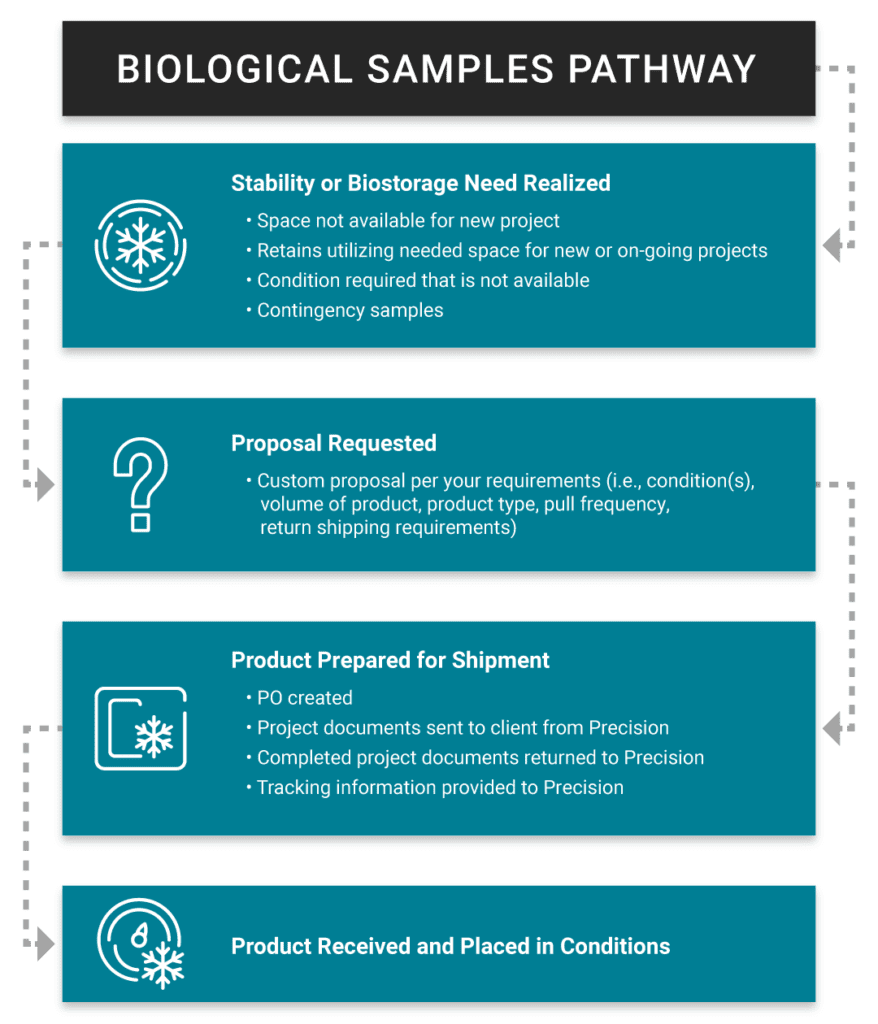
Contract Off-Site cGMP Biostorage & Biorepository Services
Precision supports the early research and pharmaceutical end products of the cold chain by offering contract off-site cGMP biostorage and biopharma storage facilities along with biological sample management. Our goal is to keep your samples stored in a manner that maintains their safety and integrity. We have multiple locations in targeted geographical regions. Our biorepositories have a variety of cGMP storage conditions readily available from ultra-cold freezer storage, refrigerated storage, ambient storage, ICH stability storage, and custom conditions upon request. A key advantage for Precision Stability Storage to safely provide cGMP biological sample storage is utilizing best-in-class storage equipment. Biological sample storage conditions are verified by our 21 CFR Part 11 continuous monitoring system, and your samples are kept safe as any out-of-tolerance will result in the system reaching out to our staff with alert notifications. At PSS, we leverage our quality system with documented procedures and processes along with highly trained and qualified staff.
Precision utilizes industry-leading controlled storage chambers such as Parameter Generation and Control due to their accuracy, stability, and reliability. Customers choose PSS knowing that we will ensure temperature and humidity tolerances as we fully understand the importance and value of our customer’s products and samples.
Types of Biological Sample Storage and Other Commonly Stored Materials:
-
- Biospecimens
- Active Pharmaceutical Ingredients (API)
- Clinical trial therapeutics
- Clinical study samples
- Controlled Raw Materials
- Bioanalytical samples
- Biopharmaceutical material
- Biomarkers
- Drug compounds
- Medical Devices
- Stability Study Samples
- Vaccines
- Other biologic materials as required
Why Outsource cGMP Biostorage to Precision Stability Storage?
-
- Space limitations at your site and overflow. Some sites would rather have more scientists running studies rather than dedicating large amounts of infrastructure to storage.
- Facility limitations at your site. Site may be at capacity, yet more storage is necessary.
- Long-term storage. There are cost advantages of utilizing an existing company with the policies, procedures, and facility ready to store to meet industry expectations
- Emergency services where your site may need a local biostorage facility that can quickly accept your products and samples.
- Geographic separation for safety and control. This is often called mirror banking to ensure if a natural disaster were to occur that your most important samples have geographic separation.
- Transitional storage. Your site may be moving or undergoing changes and you need a temporary location to store your products and samples.
How Our Biorepository Services Adhere to Best Practices
Through our experience within the biostorage industry we leverage many of the available best practices and guidance available. Our FDA and cGMP-compliant quality system has been developed and implemented over time. Precision Stability Storage staff is trained and approved based on written procedures.
Best Standards and Guideline Resources
- ISBER – International Society for Biological Environmental Repositories
- WHO – World Health Organization
- USP – US Pharmacopeia
- FDA – U.S. Food and Drug Administration
- CAP – College of American Pathologists
- ICH – International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
Quality system
PSS understands the value you would be entrusting with us, and therefore acknowledge that our customers will audit our facility. This would of course include reviewing our documentation, standard operating procedures, and general practices so any questions can be alleviated and trust earned.
- cGDP – Current Good Documentation Practice
- GDP – Good Distribution Practice
- cGMP/cGLP – Current Good Manufacturing Practice / Current Good Laboratory Practice
- Training records
- Equipment qualification (IQ/OQ/PQ)
- Continuous Monitoring System
- Validated and 21 CFR Part 11 compliant
- Monitoring and records
- Alarms and audit trails
- Record retention
- Audit and inspection support
- Continuous monitoring system, access, and inventory control
- Audit and inspection support
- Inventory system
- Location and lot control with temperature and humidity tracking records
Redundancies and backups
- Generator
- Alternative storage units
- Redundant compressors and conditioners
- Safety measures for different products stored
- Emergency response planning
Security
- Access policy
- Shipping / Receiving

