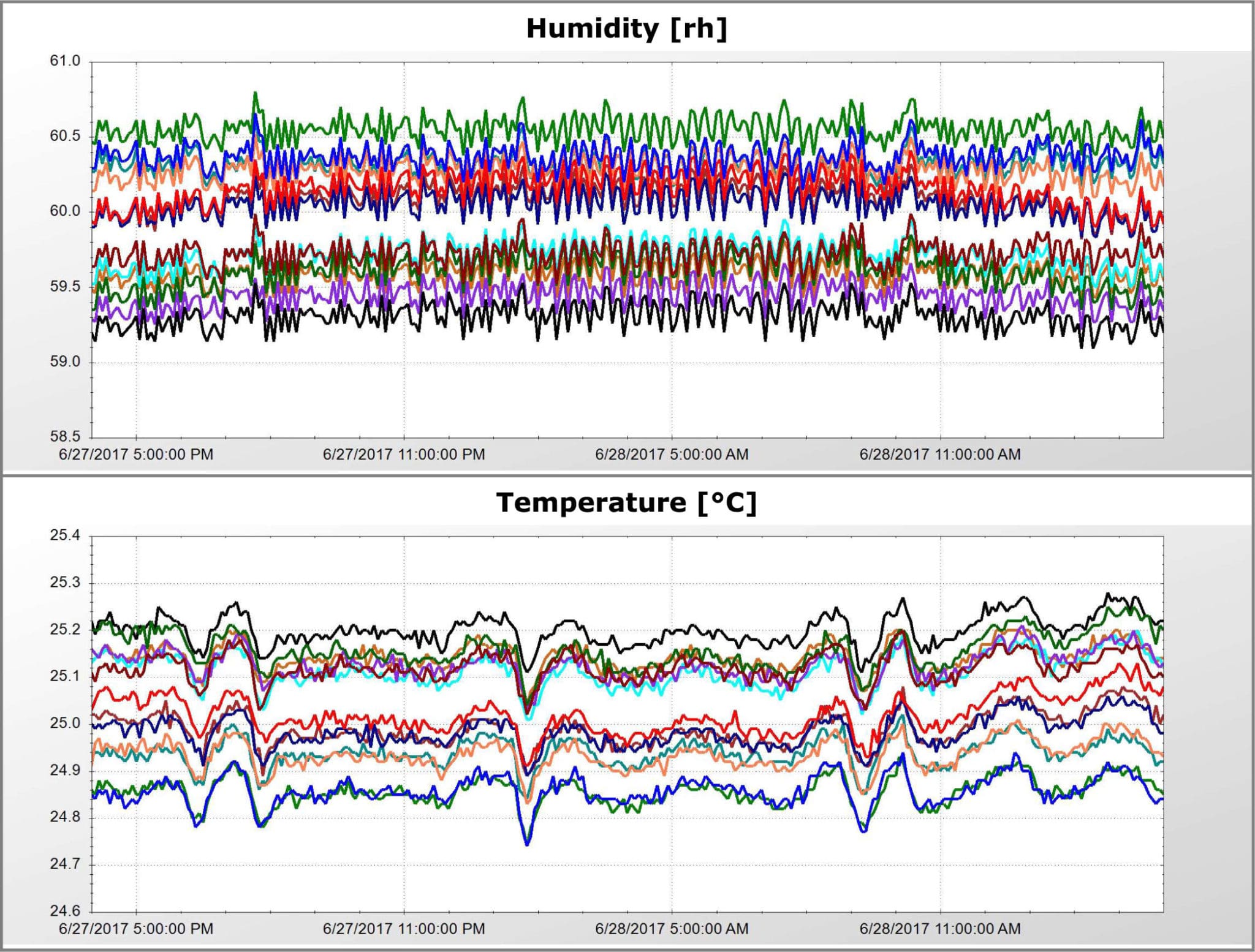
Precision Stability Storage provides off-site stability storage space for pharmaceutical products that require ICH specified environmental conditions. We offer the highest-quality equipment and facilities available for temperature and humidity control.
All of our rooms meet or exceed ICH guidelines and have been mapped and validated for FDA mandated long and short-term shelf life studies under various temperature and humidity requirements. This includes intermediate testing and accelerated testing per ICH Q1A (R2).
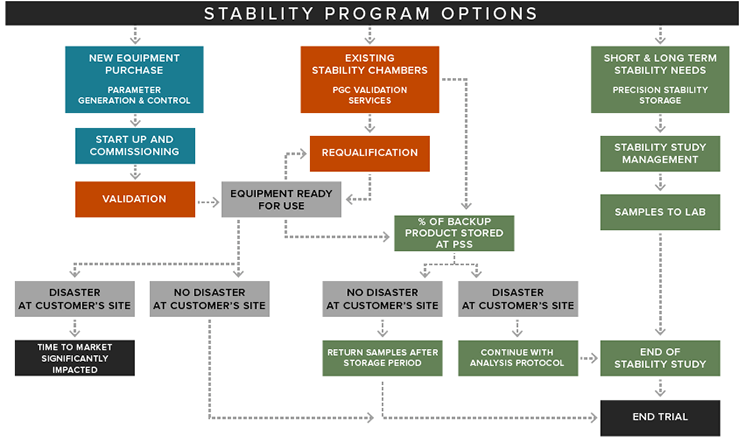
Our strategic partnership with Parameter Generation & Control allows us to offer Precision clients the highest-quality equipment and facilities available for environmental and humidity control for companies testing their new drug products.
Precision takes a proactive approach to excursions, our monitoring system provides multi-level alarming with text and e-mail out notifications allowing us to address most would be excursions before they happen. Team communication determines mitigation solution and path forward.
In the unlikely event of an excursion, our 21 car part 11 compliant equipment monitoring software provides an insight to the reason of excursion enabling us to minimize out of specification time. All excursions are addressed under Precision cGMP quality system.