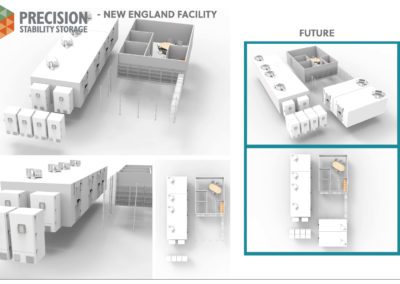
Hopedale, Massachusetts Stability Storage Facility
Our second facility in Hopedale, MA opened its doors in 2018.
This facility is strategically located for easy access to the Pharmaceutical/Biological companies of Boston area. Our location is also convenient to companies in Rhode Island, Connecticut, New York and New Jersey.
The Hopedale Facility specializes in ICH Q1A (R2) contract stability storage for Accelerated, Refrigerated, Ambient storage and -80°C Freezer requirements for the Biotech and Genetics companies. This facility has plenty of capacity to expand to various stability storage conditions as needed.
The Hopedale facility is committed to providing cGMP biostorage services, meeting the Good Manufacturing Practice (cGMP) standards and regulatory requirements for the safe storage of biological materials.
Here are some key features of the Hopedale facility:
- Back-up Conditioners and Freezers
- Back-up electrical generator to power full facility if there is a power outage
- Part 11 compliant monitoring system with alarms
- Alarmed building with proper levels of security