CALIFORNIA
Certifications:

ISO 9001:2015 (C2022-03794) – Quality Management System:
- Demonstrates our dedication to maintaining a robust quality management system to meet customer and regulatory requirements.

Licenses:
Specialized certification demonstrating our expertise in safely and securely storing biological materials.

State of California Department of Health Tissue License (CTB 00082346)

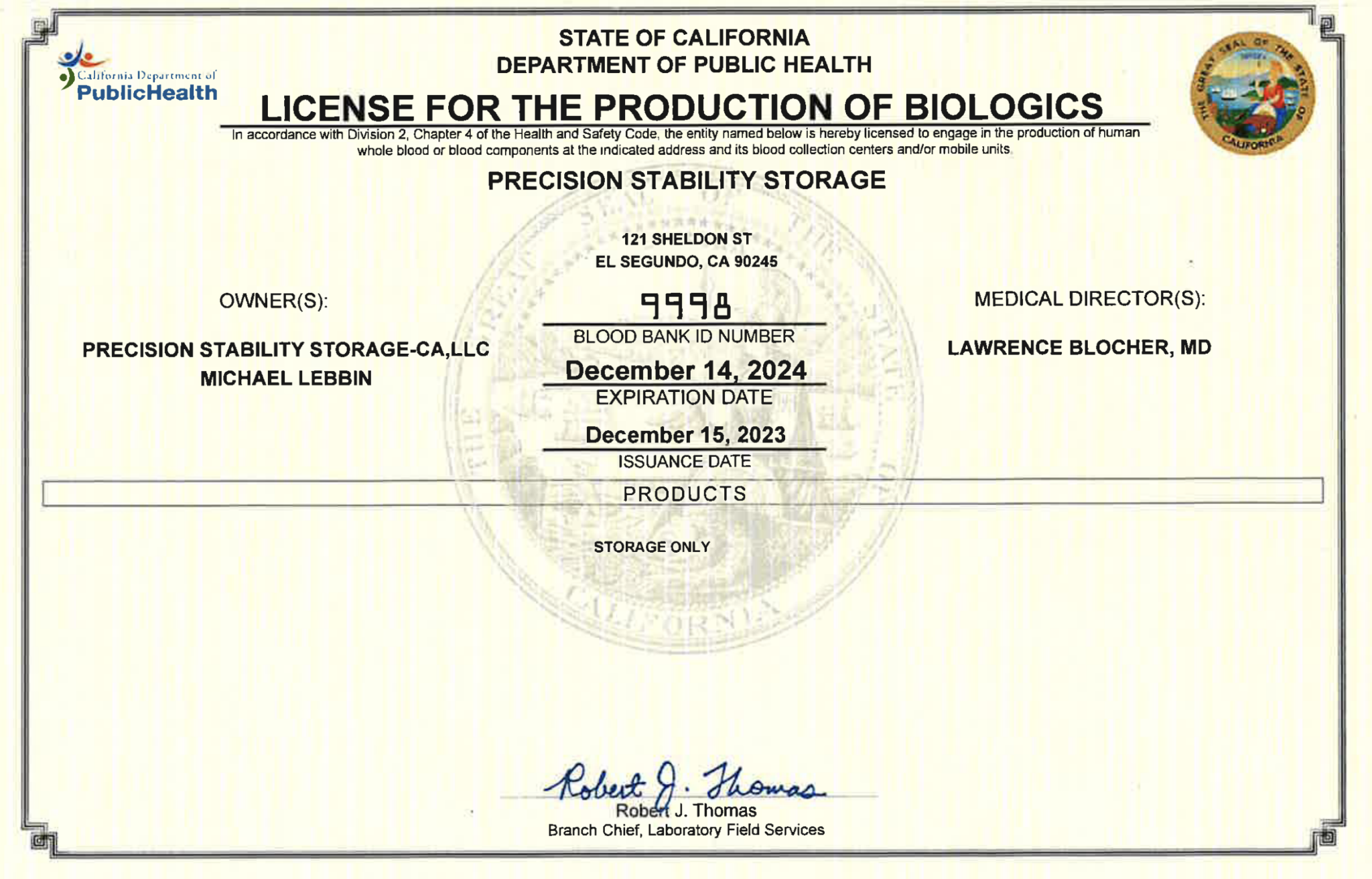
State of California Department of Health Biologics License (CBL-9998)
Registrations:

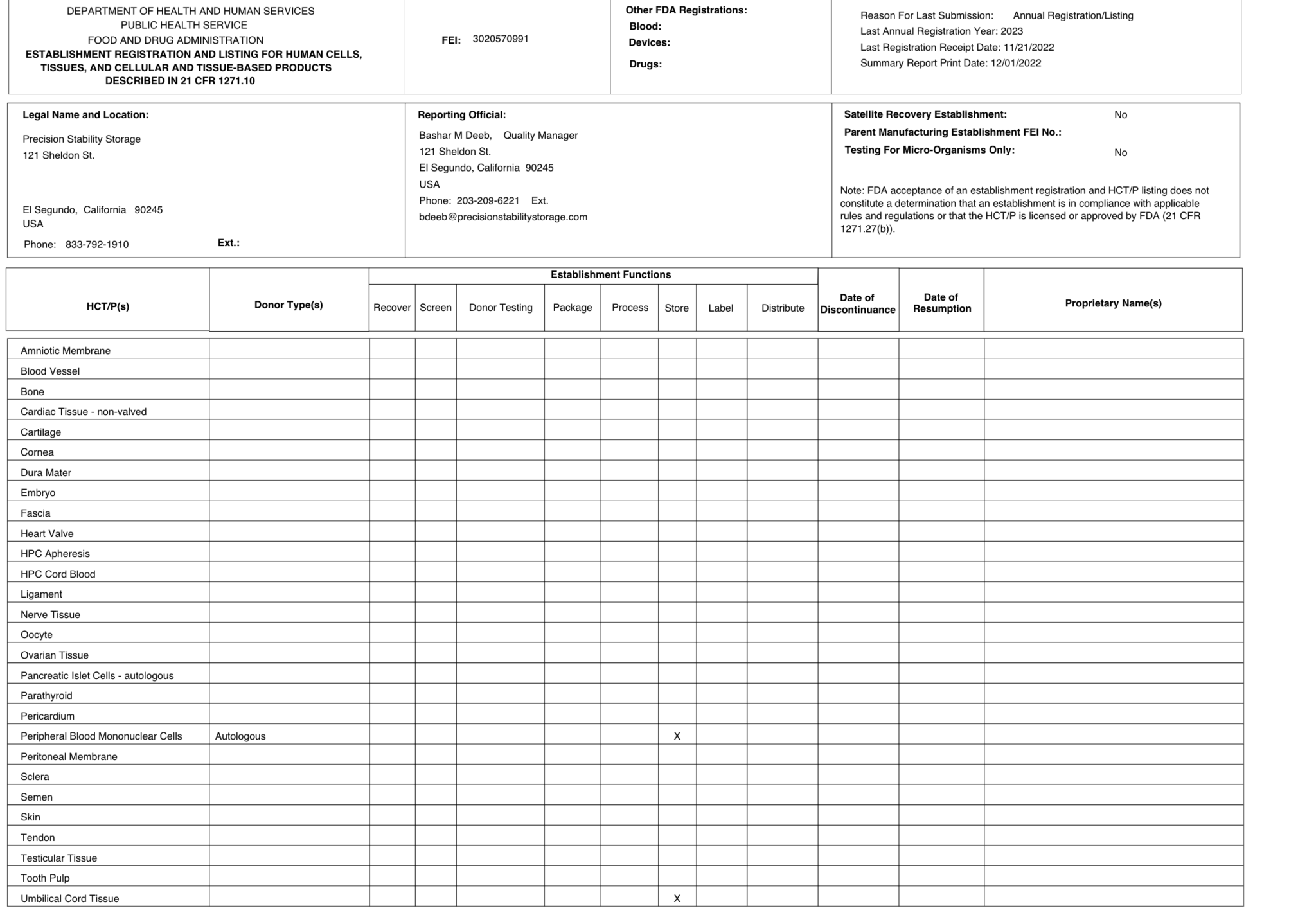
FDA registered Tissue License (3020570991)
- As a registered facility with the U.S. Food and Drug Administration, we comply with regulatory requirements for storing biological products.
FLORIDA
Certifications:

ISO 9001:2015 (C2022-03459) – Quality Management System
- Demonstrates our dedication to maintaining a robust quality management system to meet customer and regulatory requirements.

Registrations:

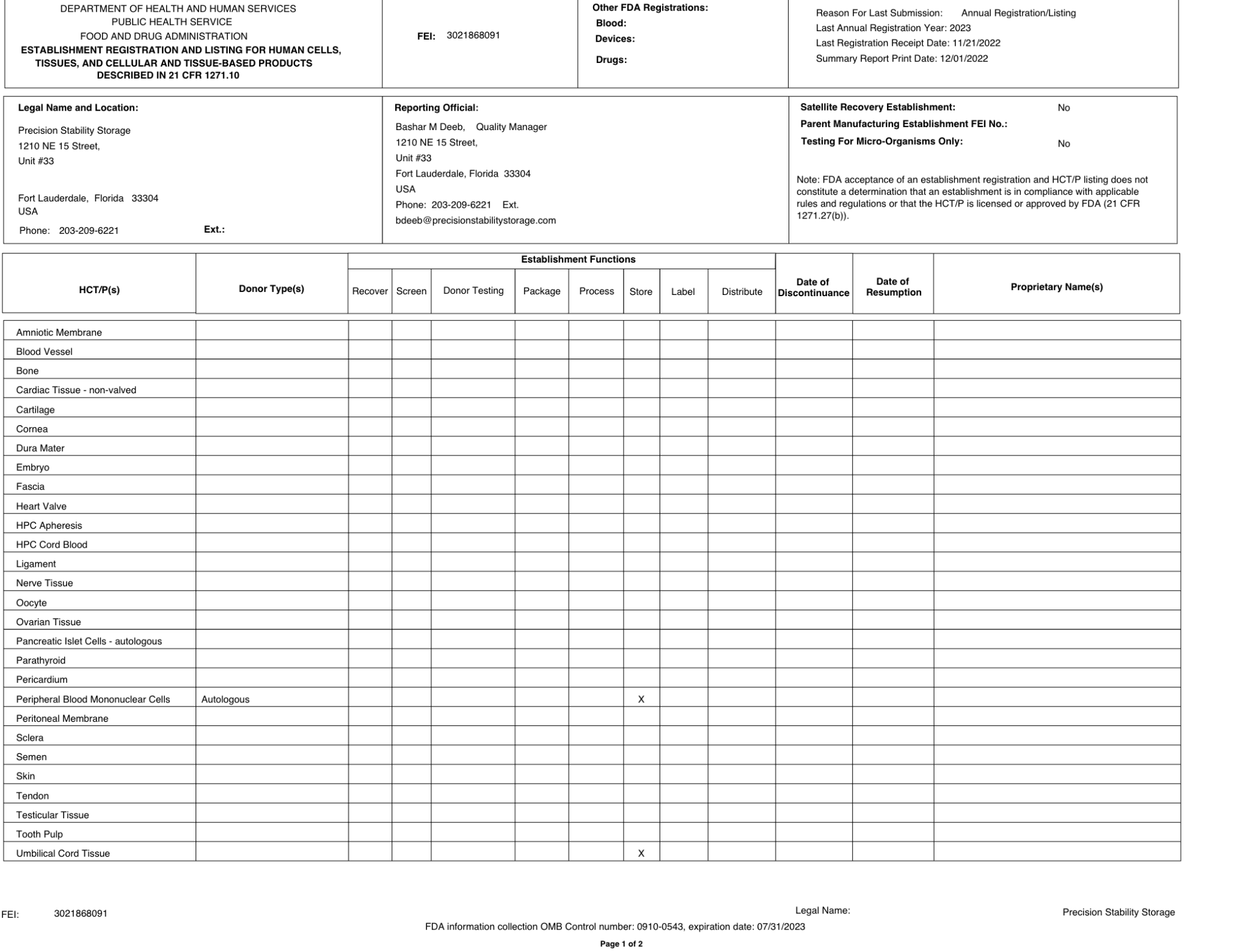
FDA registered Tissue License (3021868091)
- As a registered facility with the U.S. Food and Drug Administration, we comply with regulatory requirements for storing biological products.
MASSACHUSETTS
Certifications:

ISO 9001:2015 (C2022-05435) – Quality Management System
- Demonstrates our dedication to maintaining a robust quality management system to meet customer and regulatory requirements

Registrations:

FDA registered Tissue License (3016832701)
- As a registered facility with the U.S. Food and Drug Administration, we comply with regulatory requirements for storing biological products.

NORTH CAROLINA
We undergo audits that are compliant with ISO 9001:2015, 21 CFR 210, 211, and 820 standards.
Registrations:

FDA Registered – 3007855582
- As a registered facility with the U.S. Food and Drug Administration, we comply with regulatory requirements for storing pharmaceuticals and biological products.
Specialized Certifications:
ICH Q1A Stability Testing Compliance:
- Ensures that our stability storage conditions align with the guidelines outlined in ICH Q1A for stability testing.
GXP Regulations:
GMP (Good Manufacturing Practice):
- Adhering to GMP guidelines per customers’ requirements, ensuring our facilities and processes consistently produce and store products to the highest quality standards.
GCP (Good Clinical Practice):
- Adhering to GCP guidelines per customers’ requirements, ensuring clinical trial participants’ safety, rights, and well-being and credibility of clinical trial data.
GTP (Good Tissue Practice):
- Adhering to GTP guidelines per customers’ requirements, aiming to ensure the quality and safety of human tissue used for transplantation, protecting donors and recipients.
GLP (Good Laboratory Practice):
- Adhering to GLP guidelines per customers’ requirements, ensuring the reliability and integrity of non-clinical laboratory studies. Setting forth guidelines for conducting studies and documenting results while providing a framework for regulatory acceptance of data.













