Comprehensive Off-site cGMP Biostorage Solutions
Precision Stability Storage delivers cGMP-compliant off-site biostorage solutions tailored for research, clinical, and therapeutic applications requiring precise temperature environments. With strategically positioned facilities in California, Florida, Massachusetts, and North Carolina, we offer scalable storage options—from ambient to cryogenic conditions—ensuring material integrity and regulatory compliance. Our flexible short- and long-term storage capabilities are designed to safeguard critical assets throughout their lifecycle, providing a reliable solution to meet the stringent demands of biopharma and research industries.
Whether storing temperature-sensitive compounds, biological samples, or clinical materials, our state-of-the-art facilities ensure optimal environmental conditions for maintaining material integrity. The temperature solutions we offer include:
- Ambient Storage
- Refrigerated Storage
- Freezer Storage
Types of Biological Sample Storage & Other Commonly Stored Materials:
Secure, Compliant, and Scalable Storage for Research, Clinical and Therapeutic Products
SAFETY AND SECURITY
- 24/7/365 security system.
- Multi-level-controlled access to the main building, storage areas, and individual chambers.
- 21 CFR Part 11 compliant monitoring system with remote notifications to alert personnel of alarms.
CRITICAL REDUNDANCIES
- Backup generator capable of powering the entire facility in case of a power outage.
- Backup conditioners and at-temperature freezers and refrigerators.
- Spare parts in inventory for critical equipment.
- Equipment maintained to manufacturer specifications.
REGULATORY COMPLIANCE
- cGMP-compliant storage.
- Walk-in stability chambers meet ICH guidelines.
- Quality System with procedures for all critical operations.
- ISO 9001:2015 Certified (CA, FL, and MA facilities).
- FDA Registered (CA, FL, MA, and NC)
Learn more about our License, Registrations, and Certifications.
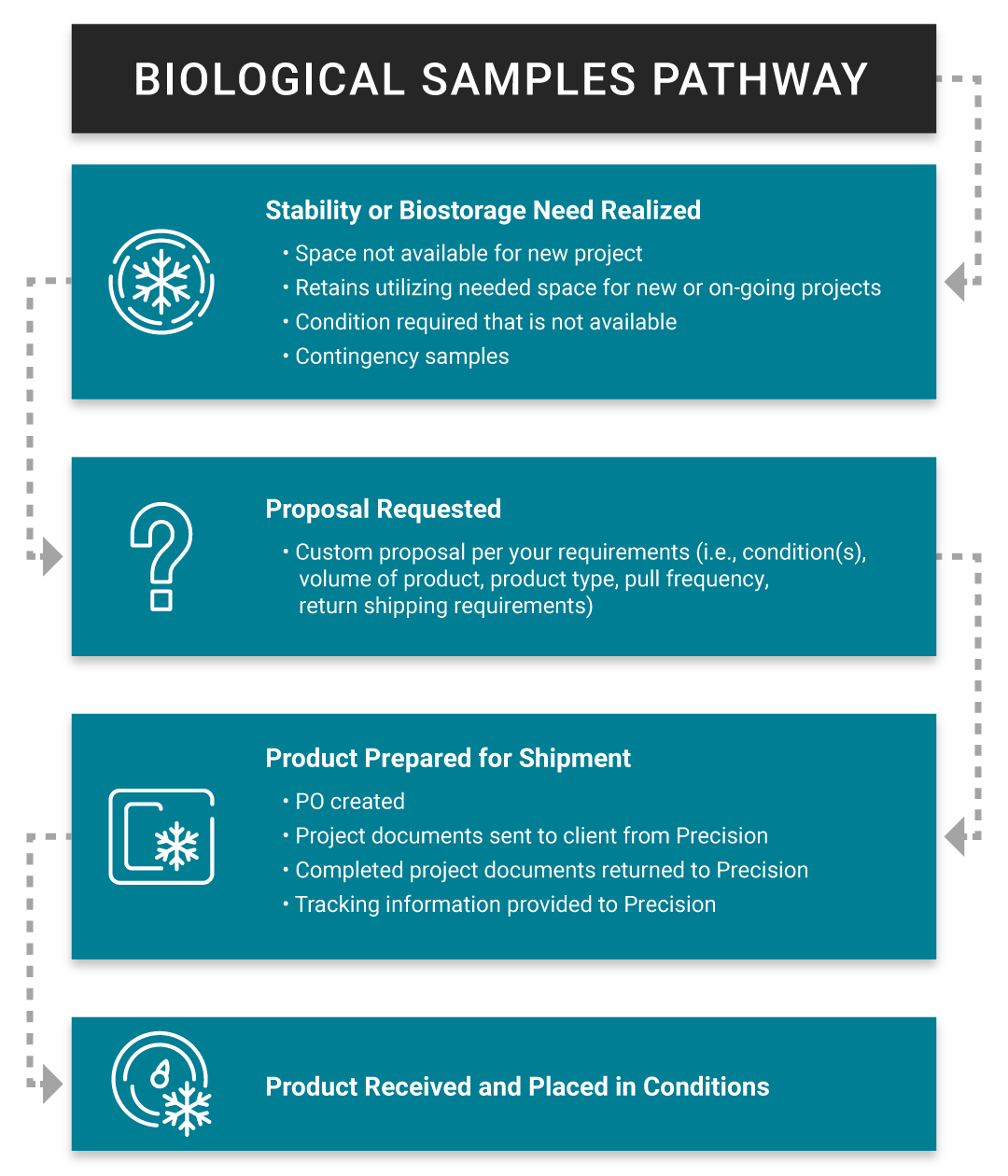
Why Outsource cGMP Biostorage to Precision Stability Storage?
- Reduce overhead costs: Outsourcing biostorage helps eliminate the need to maintain expensive on-site storage infrastructure, reduce operational expenses, and redirect resources towards core research or product development activities.
- Facility capacity challenges: When storage needs exceed a site’s available space, it can create operational challenges. Offsite storage offers a flexible, scalable solution to manage the growing demand for secure, compliant storage.
- Geographic risk mitigation: Storing critical materials and products in multiple locations ensures their protection from regional risks, such as natural disasters, providing added security through geographic separation.
- Emergency storage solutions: When immediate storage is required, Precision offers fast, reliable access to secure facilities, ensuring critical materials and products are stored without delay.
- Temporary storage options: During transitions or unexpected changes, we offer flexible, short-term storage solutions, ensuring research continuity and operations.
- Industry-leading certifications: PSS holds key industry certifications, including ISO 9001 and cGMP compliance, which ensure that all storage processes meet the highest standards of quality, safety, and regulatory adherence. Learn more about our certifications here.
- Biospecimens
- Active Pharmaceutical Ingredients (API)
- Clinical trial therapeutics
- Clinical study samples
- Controlled Raw Materials
- Bioanalytical samples
- Biopharmaceutical material
- Biomarkers
- Drug compounds
- Medical Devices
- Stability Study Samples
- Vaccines
- Other biologic materials as required
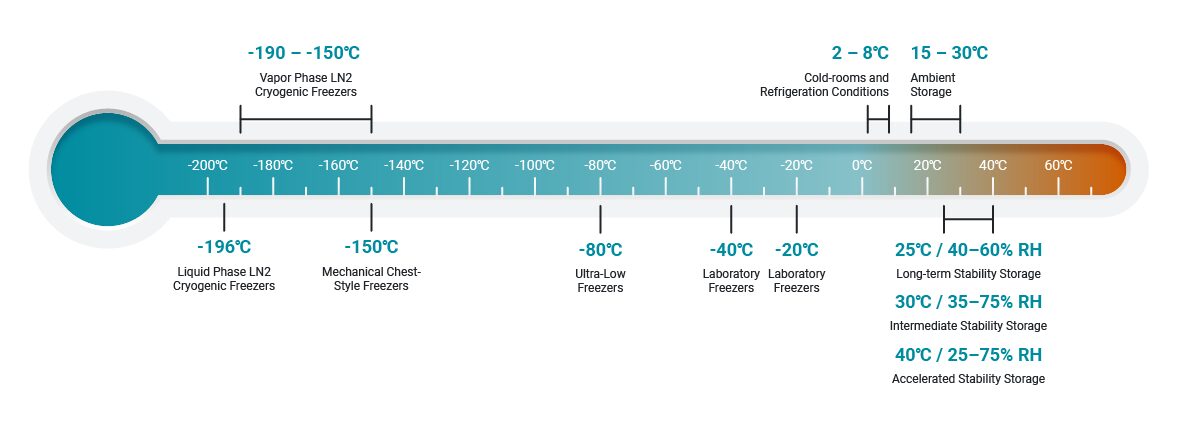
Our Range of Biostorage Temperature Solutions
Whether you’re storing temperature-sensitive compounds, biological samples, or clinical materials, our state-of-the-art facilities ensure optimal environmental conditions for maintaining product integrity. The temperature solutions we offer include:
- Stability Storage
- Ambient Storage
- Refrigerated Storage
- Freezer Storage
- Ultra-Low Freezer Storage
- Cryogenic Storage
Designed for long-term storage under controlled conditions. Ideal for testing stability over time, we offer storage solutions ranging from +20°C to +25°C, ensuring consistent and reliable results for pharmaceutical and research products.
Suitable for materials that remain stable at room temperature (15°C to 25°C), such as paraffin-embedded tissue samples, DNA, RNA, proteins, and other biomolecules preserved with fixatives.
Perfect for samples and products that require a colder, but not freezing, environment. Our refrigerated storage maintains precise temperatures between +2°C to +8°C, commonly used for vaccines, chemicals, and biologics.
For materials that need to be stored at sub-zero temperatures, our freezer storage solutions range from -20°C +/- 5, providing an excellent option for a wide variety of biological and chemical samples.
When you need extreme cold storage, our ultra-low freezers operate at temperatures around -80°C, offering ideal conditions for long-term storage of sensitive biological samples such as enzymes, RNA, or certain cell types.
For the most delicate materials, including cellular therapies and reproductive tissues, our cryogenic storage solutions maintain temperatures at or below -190°C. This ensures that even the most fragile specimens remain viable during storage.
Industries Our cGMP Biostorage Facilities Serve
How Our cGMP Biostorage & Biorepository Services Adhere to Best Practices
cGMP Biostorage Resources
- ISBER – International Society for Biological Environmental Repositories
- WHO – World Health Organization
- USP – US Pharmacopeia
- FDA – U.S. Food and Drug Administration
- CAP – College of American Pathologists
- ICH Guidelines – International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
Quality System
At Precision, we recognize the trust our customers place in us when storing their valuable materials. We welcome facility audits and provide full transparency through detailed documentation, standard operating procedures (SOPs), and best practices. Our commitment to quality ensures all questions are addressed, fostering confidence and building long-term partnerships.
- cGDP – Current Good Documentation Practice
- GDP – Good Distribution Practice
- cGMP/cGLP – Current Good Manufacturing Practice / Current Good Laboratory Practice
- Training records
- Equipment qualification (IQ/OQ/PQ)
- Continuous Monitoring System
- Validated and 21 CFR Part 11 compliant
- Real-time monitoring and records
- Alarms and audit trails
- Comprehensive Record Retention – Maintain accurate and organized records to support compliance and traceability.
- Audit and Inspection Readiness – Ensure seamless audit preparation with complete documentation and procedural transparency.
- Robust Inventory Management
- Track and manage assets and associated data in a software-based system.
- Maintain temperature and humidity records with accurate lot and location tracking for compliance and quality assurance
FDA Regulations and Compliance
Precision Stability Storage (PSS) adheres to stringent FDA regulations, ensuring that all stored materials meet the highest safety and compliance standards required for cGMP biostorage. Our cGMP storage facilities are designed to fully comply with these regulations, guaranteeing that your samples are stored under the most secure and compliant conditions.
