Across the world, climate zones represent diverse environmental conditions, each with unique temperature and humidity profiles. This variety in climates plays a significant role in the stability and efficacy of pharmaceutical products.
Understanding how these varying climatic conditions can affect drug characteristics is crucial in the pharmaceutical industry to ensure that medications remain safe and effective regardless of where they are stored or used.
Read on to learn more about climate zones and the role they play in stability testing.
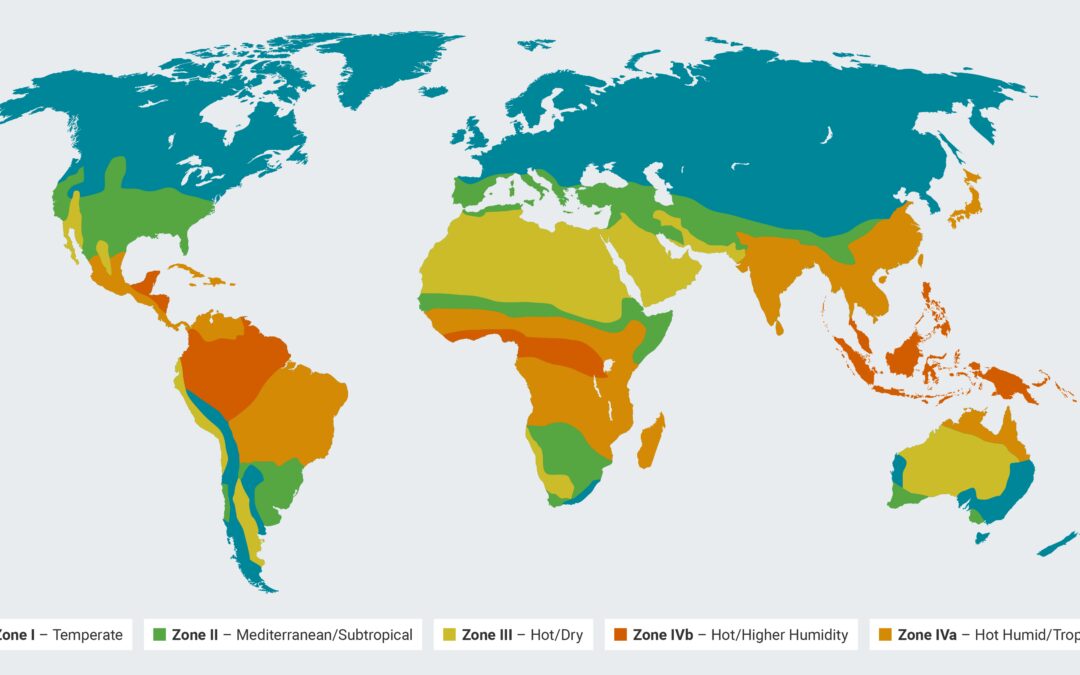
WHAT IS AN ICH CLIMATIC ZONE?
An ICH Climatic Zone refers to a classification system established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). This system categorizes different parts of the world into specific zones based on their prevailing climatic conditions.
The classification of a climatic zone is determined primarily by two key environmental factors: temperature and humidity. These factors are critical in understanding how pharmaceutical products react over time in different geographic locations.
This categorization aids in developing global standards for drug safety and efficacy, facilitating the harmonization of pharmaceutical regulations across different countries.
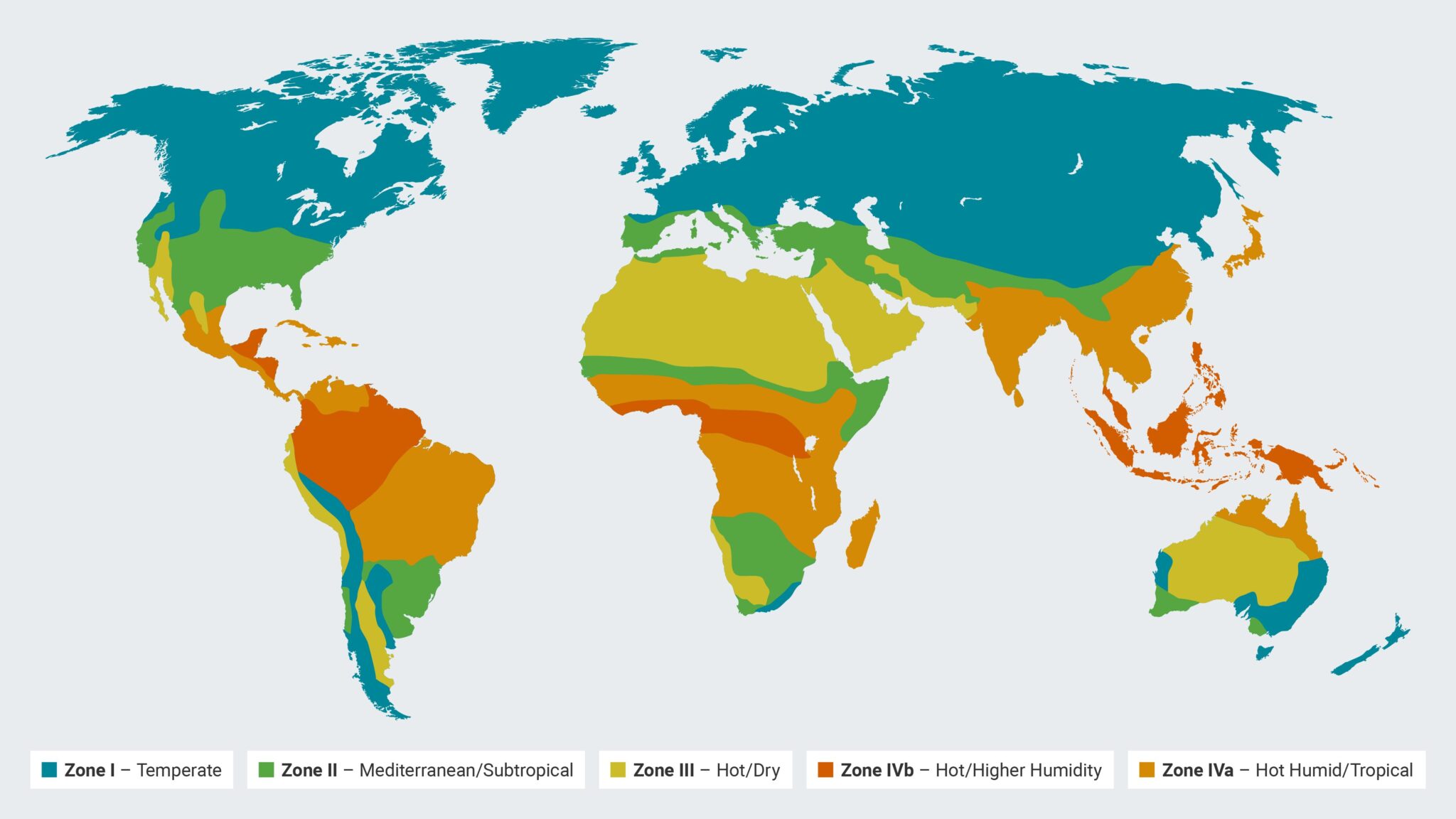
WHAT ARE THE 4 TYPES OF ICH STABILITY ZONES?
The International Council for Harmonisation (ICH) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs.
These zones represent different climatic conditions worldwide to ensure that pharmaceutical companies test products under environmental conditions similar to where they will be stored and used.
Each zone has specific temperature and humidity conditions that simulate the climatic environment of various geographic regions.
Learn more about the four ICH Stability Zones below:
| Zone | Description | Temperature | Relative Humidity | Geographic Areas |
| I | Temperate Zone | 21°C | 45% RH | Southern Canada, Europe, parts of Russia |
| II | Mediterranean/Subtropical Zone | 25°C | 60% RH | Mediterranean region, parts of Australia, southern USA |
| III | Hot/Dry Zone | 30°C | 35% RH | North Africa, Middle East, desert areas in the USA |
| IVa | Hot Humid/Tropical Zone | 30°C | 65% RH | Southeast Asia, Central Africa, parts of South America |
| IVb | Hot/Higher Humidity Zone | 30°C | 75% RH | Regions near the equator, dense rainforest areas |
ZONE I: TEMPERATE ZONE
The Temperate Zone, designated as Zone I by the International Council for Harmonisation (ICH), contains regions with a mild and moderate climate.
Characteristically, these areas experience four distinct seasons, with relatively mild winters and summers. For pharmaceutical stability testing, the typical environmental conditions of this zone are at 21°C with 45% relative humidity (RH).
Geographically, this zone includes much of Southern Canada, most of Europe, and parts of Russia, characterized by their temperate maritime and continental climates.
ZONE II: MEDITERRANEAN/SUBTROPICAL ZONE
Zone II, known as the Mediterranean or Subtropical Zone, refers to areas where the climate is generally warmer than the Temperate Zone. It features hot, dry summers and cool, wet winters.
Standard conditions for stability testing in this zone are 25°C with 60% RH. Zone II covers areas like the Mediterranean region, parts of Australia, and the southern coastal regions of the USA, where the climate is significantly influenced by warm ocean currents.
ZONE III: HOT/DRY ZONE
Zone III experiences high temperatures and low humidity, typical of desert and semi-arid regions. For stability testing, the conditions are set at 30°C with 35% RH, reflecting the challenging dry and hot environment.
Geographically, this includes large parts of North Africa, the Middle East, and desert areas in the United States like Arizona and Nevada, where the climate is predominantly arid.
ZONE IVA: HOT HUMID/TROPICAL ZONE
Zone IVa is characterized by consistently high temperatures and significant humidity throughout the year. The standard testing conditions for pharmaceutical products in this zone are 30°C with 65% RH.
This zone includes regions like Southeast Asia, Central Africa, and parts of South America, where the climate is typically tropical with high rainfall and humidity levels.
ZONE IVA: HOT/HIGHER HUMIDITY ZONE
Zone IVb, or the Hot/Higher Humidity Zone, represents areas with extreme humidity and high temperatures. It’s a subset of the tropical zone, with even more challenging environmental conditions for pharmaceutical stability.
The standard testing parameters here are 30°C with a higher humidity level of 75% RH. This zone is particularly relevant for regions close to the equator or areas with dense rainforests, like the Amazon Basin and Central Africa, where the climate is exceptionally humid and hot year-round.
WHAT ARE ICH STABILITY CONDITIONS?
This section should explain why ICH stability zones need to be studied and tested, then include a breakdown of the different stability conditions in a table format. See the competitor example below for reference:
ACCELERATED AMBIENT
The Accelerated Ambient condition in ICH stability testing speeds up the rate of chemical degradation or physical change of a pharmaceutical product at a higher temperature than standard ambient conditions.
Typically, this test is conducted at 40°C with 75% relative humidity. Accelerated ambient testing assesses the short-term stability of a product and predicts its shelf life under normal storage conditions. This accelerated testing is crucial for identifying potential stability issues early in drug development.
ACCELERATED REFRIGERATED
Accelerated Refrigerated conditions test the stability of pharmaceutical products typically stored in cool environments. This testing is typically conducted at a temperature of 5°C, higher than standard refrigeration temperatures.
The purpose is to observe how slight elevations in temperature might affect products that require refrigeration, such as certain biologics, vaccines, or other temperature-sensitive medications.
ACCELERATED FROZEN
Accelerated Frozen conditions test pharmaceutical products that must remain within freezing temperatures at higher temperatures around -10°C to -20°C, compared to the standard storage temperatures of -20°C or lower.
This testing evaluates the stability of products like certain vaccines and biological materials, ensuring they remain stable and effective even when exposed to slightly higher temperatures than their frozen state.
INTERMEDIATE
Intermediate stability testing is a step between accelerated and long-term testing conditions. It usually involves storing the pharmaceutical product at 30°C with 65% relative humidity. This condition is helpful when accelerated testing shows significant change or if long-term testing data at 25°C are needed quickly.
It provides a balance, offering a more rigorous test than long-term conditions but less extreme than accelerated ambient testing. Intermediate testing helps in understanding how a drug might perform under conditions that are neither excessively harsh nor overly mild.
CONTACT PRECISION STABILITY STORAGE TODAY!
Understanding ICH climatic zones is crucial for the pharmaceutical industry. These zones ensure that drugs are tested for stability under varied environmental conditions, mirroring the climates where they will be eventually used and stored. This rigorous testing is essential for maintaining the safety, efficacy, and quality of pharmaceutical products across the globe.
Precision Stability Storage provides specialized ICH stability storage solutions that cater to these diverse climatic requirements. Our state-of-the-art off-site facilities replicate the specific conditions of each ICH zone to guarantee accurate and compliant stability testing for your pharmaceutical products.
For reliable and precise stability storage services, contact us for more information. Request a quote today and ensure your products meet the highest standards of global pharmaceutical compliance and quality.